Sports
Health
When Health Disclosures Become Campaign Issues | The Report
John F. Kennedy was a sick man, however hardly anybody knew it, because the late president saved his well being...
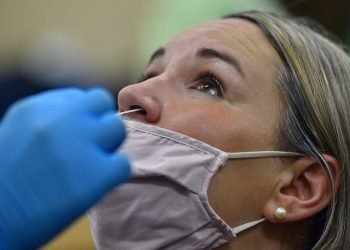
Public Health Officials Continue to Report Slide in COVID Numbers, with 1,591 for Week
A Mira Mesa Excessive Faculty administrative assistant examined for COVID 19 in 2021. Picture by Chris Stone San Diego County...
Long Beach Library reopens after month-long closure due to ‘mental health’ safety issues
The Billie Jean King Foremost Library in Lengthy Seashore reopened to guests Thursday after being closed for roughly a month...
Over 5,000 Patients Have Worked with NYC Health + Hospital Community Health Workers, Who Address Patients’ Pressing Social Needs To Improve Their Health
Over 5,000 Sufferers Have Labored With NYC Well being + Hospital Group Well being Employees, Who Handle Sufferers’ Urgent Social...
McLeod Health Welcomes These New Physicians
McLEOD HEALTH LORIS 20 OCTOBER 2022 McLeod Well being welcomes Dr. Morganne B. Beard and Dr. Hans E. Blunck to...
Jail officials across Pa. sound alarm as mental health crisis puts people at risk, survey finds · Spotlight PA
Spotlight PA is an impartial, nonpartisan newsroom powered by The Philadelphia Inquirer in partnership with PennLive/The Patriot-Information, TribLIVE/Pittsburgh Tribune-Evaluation, and...